Let's talk waxing
#76
Keefusb
Join Date: Jan 2021
Location: Ashland, VA
Posts: 176
Bikes: 60cm 1992 Paramount, 60cm 1995 Cannondale R900 (son's bike), 1994 Cannondale H300 (mine), 1994 Cannondale H300 Killer V (wife's bike), 60 cm 1989 Eddy Merckx Corsa Extra SLX
Mentioned: 0 Post(s)
Tagged: 0 Thread(s)
Quoted: 83 Post(s)
Liked 57 Times
in
36 Posts
From a physical chemistry perspective, the mineral oil lowers the specific heat and melting temperature of paraffin wax. It also makes the molten paraffin less viscous. It would stand to reason that by thinning the liquid paraffin, mineral oil + paraffin would have a greater capacity to flow into tight spaces and displace air like the gaps between the side plates, pins, and rollers.
I don't have the instruments to quantitatively measure the before and after viscosities, nor do I have the ability to measure the before and after heat capacities. I do know that the mineral oil + paraffin mix melts at lower temperature than just paraffin, which would support the point that mineral oil lowers the specific heat of plain old paraffin.
Again, former chemist and chemistry major from an accredited, nationally-ranked, brick-and-mortar university speaking.
I don't have the instruments to quantitatively measure the before and after viscosities, nor do I have the ability to measure the before and after heat capacities. I do know that the mineral oil + paraffin mix melts at lower temperature than just paraffin, which would support the point that mineral oil lowers the specific heat of plain old paraffin.
Again, former chemist and chemistry major from an accredited, nationally-ranked, brick-and-mortar university speaking.
Last edited by Keefusb; 10-02-23 at 12:00 PM.
Likes For Keefusb:
#77
Senior Member
Join Date: Apr 2011
Posts: 7,067
Mentioned: 41 Post(s)
Tagged: 0 Thread(s)
Quoted: 4409 Post(s)
Liked 1,566 Times
in
1,028 Posts
From a physical chemistry perspective, the mineral oil lowers the specific heat and melting temperature of paraffin wax. It also makes the molten paraffin less viscous. It would stand to reason that by thinning the liquid paraffin, mineral oil + paraffin would have a greater capacity to flow into tight spaces and displace air like the gaps between the side plates, pins, and rollers.
I don't have the instruments to quantitatively measure the before and after viscosities, nor do I have the ability to measure the before and after heat capacities. I do know that the mineral oil + paraffin mix melts at lower temperature than just paraffin, which would support the point that mineral oil lowers the specific heat of plain old paraffin.
Again, former chemist and chemistry major from an accredited, nationally ranked university speaking.
I don't have the instruments to quantitatively measure the before and after viscosities, nor do I have the ability to measure the before and after heat capacities. I do know that the mineral oil + paraffin mix melts at lower temperature than just paraffin, which would support the point that mineral oil lowers the specific heat of plain old paraffin.
Again, former chemist and chemistry major from an accredited, nationally ranked university speaking.
#78
ignominious poltroon
Join Date: Jan 2022
Posts: 4,046
Mentioned: 3 Post(s)
Tagged: 0 Thread(s)
Quoted: 2241 Post(s)
Liked 3,443 Times
in
1,802 Posts
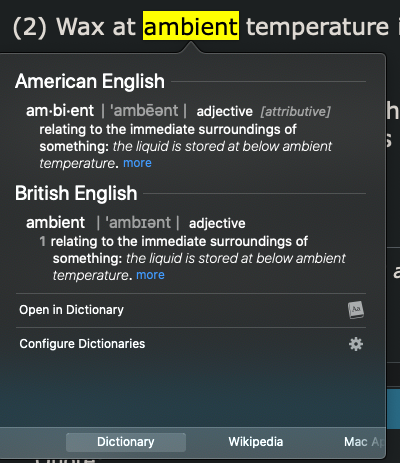
(2) Wax at ambient temperature is not a liquid in the conventional sense.
I haven't tried mineral oil, but I have mixed mineral spirits with wax, which makes it less brittle in the solid phase. However, I found the mixture did not last as long.
Last edited by Polaris OBark; 10-03-23 at 10:09 PM.
#79
Senior Member
Join Date: Apr 2011
Posts: 7,067
Mentioned: 41 Post(s)
Tagged: 0 Thread(s)
Quoted: 4409 Post(s)
Liked 1,566 Times
in
1,028 Posts
Wax at ambient isn't a solid?
#80
ignominious poltroon
Join Date: Jan 2022
Posts: 4,046
Mentioned: 3 Post(s)
Tagged: 0 Thread(s)
Quoted: 2241 Post(s)
Liked 3,443 Times
in
1,802 Posts
Not quite. Have you ever noticed what happens to old decorative candles (ones that never see a flame)?
#81
Senior Member
Join Date: Apr 2011
Posts: 7,067
Mentioned: 41 Post(s)
Tagged: 0 Thread(s)
Quoted: 4409 Post(s)
Liked 1,566 Times
in
1,028 Posts
However, I don't think wax moves fast enough in its solid state to matter for our purposes. My 15 year old bricks of candle wax I use on chains certainly haven't changed shape in their packages.
So I would say that wax at ambient isn't a liquid at all.
#82
Senior Member
If liquid evaporation that happened at ambient was "boiling", then there wouldn't be any such thing as a "boiling point." This would come as a surprise to cooks, given the long history of people using the boiling point as a readily-available control mechanism for the temperature of hot water...
Depending on the semantic context, "boiling implies evaporation" might be true. But "evaporation implies boiling" generally is not.
Likes For HTupolev:
#83
Administrator
Join Date: Feb 2005
Location: Delaware shore
Posts: 13,558
Bikes: Cervelo C5, Guru Photon, Waterford, Specialized CX
Mentioned: 16 Post(s)
Tagged: 0 Thread(s)
Quoted: 1106 Post(s)
Liked 2,180 Times
in
1,470 Posts
I just cleaned up the thread. Let’s keep responses to reasonable discussions about the topic and avoid insults.
Likes For StanSeven:
#84
ignominious poltroon
Join Date: Jan 2022
Posts: 4,046
Mentioned: 3 Post(s)
Tagged: 0 Thread(s)
Quoted: 2241 Post(s)
Liked 3,443 Times
in
1,802 Posts
#85
Mad bike riding scientist
Join Date: Nov 2004
Location: Denver, CO
Posts: 27,366
Bikes: Some silver ones, a red one, a black and orange one, and a few titanium ones
Mentioned: 152 Post(s)
Tagged: 1 Thread(s)
Quoted: 6219 Post(s)
Liked 4,220 Times
in
2,367 Posts
Evaporation can occur at lower temperatures and is a surface phenomena.
However, I don't think wax moves fast enough in its solid state to matter for our purposes. My 15 year old bricks of candle wax I use on chains certainly haven't changed shape in their packages.
So I would say that wax at ambient isn't a liquid at all.
So I would say that wax at ambient isn't a liquid at all.
__________________
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Last edited by cyccommute; 10-04-23 at 06:18 PM.
#86
Mad bike riding scientist
Join Date: Nov 2004
Location: Denver, CO
Posts: 27,366
Bikes: Some silver ones, a red one, a black and orange one, and a few titanium ones
Mentioned: 152 Post(s)
Tagged: 1 Thread(s)
Quoted: 6219 Post(s)
Liked 4,220 Times
in
2,367 Posts
Most everyone would consider wax to be a solid at ambient temperature. “Ambient temperature” is something of a nebulous definition, however. Most scientists would say that “ambient” or room temperature are around 20°C (70°F). Ambient temperature in Phoenix, AZ would be higher but we like to temperature control our dwellings at “room temperature”. The other problem is that paraffin wax has a fairly low melting point…37°C (99°F). Any change in form is more likely to be from higher temperature exposure. It’s still a solid, however.
__________________
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
#87
Senior Member
Join Date: Dec 2020
Location: Wake Forest, NC
Posts: 5,795
Bikes: 1989 Cinelli Supercorsa
Mentioned: 11 Post(s)
Tagged: 0 Thread(s)
Quoted: 3513 Post(s)
Liked 2,927 Times
in
1,776 Posts
#88
ignominious poltroon
Join Date: Jan 2022
Posts: 4,046
Mentioned: 3 Post(s)
Tagged: 0 Thread(s)
Quoted: 2241 Post(s)
Liked 3,443 Times
in
1,802 Posts
Most everyone would consider wax to be a solid at ambient temperature. “Ambient temperature” is something of a nebulous definition, however. Most scientists would say that “ambient” or room temperature are around 20°C (70°F). Ambient temperature in Phoenix, AZ would be higher but we like to temperature control our dwellings at “room temperature”. The other problem is that paraffin wax has a fairly low melting point…37°C (99°F). Any change in form is more likely to be from higher temperature exposure. It’s still a solid, however.
However, the phase boundary isn't as sharp as for a pure molecule. Paraffin is a collection of linear hydrocarbons of various lengths, and the melting point, as a consequence, is quite broad (and a little bit higher than 37°, or it would melt in your hand). Its lubricating properties are probably a consequence of the indistinct phase boundary. It is a little bit like a crystal of a biological macromolecule, which is in many ways more like a highly concentrated solution that happens to be ordered in a three-dimensional array. I don't want to push that analogy too far, because paraffin crystals (which are formed during purification) are macromolecular crystals without any other solvent present. Because they are hydrocarbons, they form no electrostatic or hydrogen bond interactions, so the crystals themselves have very little structural integrity, and, again, there is a mixture of molecular weights. So waxes, and wax-like substances like polyethylene glycol, have broad, indistinct phase boundaries. Another way to see that is their diffraction properties. We used wax rings to align X-ray detectors and the beam prior to data collection; (it makes it much easier to index a diffraction pattern). The rings (which are like a powder pattern) correspond to the correlation lengths of carbon atoms between molecules, and are a standard way of characterizing phases (and identifying phase changes) for condensed-matter chemists. For wax, unlike most solids, there is no abrupt transition upon melting. I rather suspect this again is a feature that makes it a good lubricant.
#89
Mad bike riding scientist
Join Date: Nov 2004
Location: Denver, CO
Posts: 27,366
Bikes: Some silver ones, a red one, a black and orange one, and a few titanium ones
Mentioned: 152 Post(s)
Tagged: 1 Thread(s)
Quoted: 6219 Post(s)
Liked 4,220 Times
in
2,367 Posts
However, the phase boundary isn't as sharp as for a pure molecule. Paraffin is a collection of linear hydrocarbons of various lengths, and the melting point, as a consequence, is quite broad (and a little bit higher than 37°, or it would melt in your hand). Its lubricating properties are probably a consequence of the indistinct phase boundary. It is a little bit like a crystal of a biological macromolecule, which is in many ways more like a highly concentrated solution that happens to be ordered in a three-dimensional array. I don't want to push that analogy too far, because paraffin crystals (which are formed during purification) are macromolecular crystals without any other solvent present. Because they are hydrocarbons, they form no electrostatic or hydrogen bond interactions, so the crystals themselves have very little structural integrity, and, again, there is a mixture of molecular weights. So waxes, and wax-like substances like polyethylene glycol, have broad, indistinct phase boundaries. Another way to see that is their diffraction properties. We used wax rings to align X-ray detectors and the beam prior to data collection; (it makes it much easier to index a diffraction pattern). The rings (which are like a powder pattern) correspond to the correlation lengths of carbon atoms between molecules, and are a standard way of characterizing phases (and identifying phase changes) for condensed-matter chemists. For wax, unlike most solids, there is no abrupt transition upon melting. I rather suspect this again is a feature that makes it a good lubricant.
__________________
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Likes For cyccommute:
#90
Keefusb
Join Date: Jan 2021
Location: Ashland, VA
Posts: 176
Bikes: 60cm 1992 Paramount, 60cm 1995 Cannondale R900 (son's bike), 1994 Cannondale H300 (mine), 1994 Cannondale H300 Killer V (wife's bike), 60 cm 1989 Eddy Merckx Corsa Extra SLX
Mentioned: 0 Post(s)
Tagged: 0 Thread(s)
Quoted: 83 Post(s)
Liked 57 Times
in
36 Posts
I had a high school chemistry teacher who asked the class if he could boil a flask full of ice water without melting the ice. He took the flask around the room for the students to touch and feel the cold flask. He then hooked the flask to an evacuation pump and the ice water started to bubble just like if it was being heated up. So the lesson really stuck with me that "boiling point" of a substance is the temperature at which vapor pressure of a substance equals atmospheric pressure. The general assumption is that the published boiling point of a liquid is the temperature at which it's vapor pressure equals atmospheric pressure when atmospheric pressure equals 760 mm of mercury or 1 atm.
Likes For Keefusb:
#91
Method to My Madness
Join Date: Nov 2020
Location: Orange County, California
Posts: 3,663
Bikes: Trek FX 2, Cannondale Synapse, Cannondale CAAD4, Santa Cruz Stigmata GRX
Mentioned: 4 Post(s)
Tagged: 0 Thread(s)
Quoted: 1948 Post(s)
Liked 1,471 Times
in
1,020 Posts
Ah ... semantics!
If you ask a layperson, what is the opposite of boiling, the layperson would say freezing.
If you ask a scientist, what is the opposite of boiling, the scientist would say condensation.
If you ask a layperson, what is the opposite of boiling, the layperson would say freezing.
If you ask a scientist, what is the opposite of boiling, the scientist would say condensation.
Likes For SoSmellyAir:
#92
Senior Member
...There's also room for ambiguity from the word "opposite."
#93
Keefusb
Join Date: Jan 2021
Location: Ashland, VA
Posts: 176
Bikes: 60cm 1992 Paramount, 60cm 1995 Cannondale R900 (son's bike), 1994 Cannondale H300 (mine), 1994 Cannondale H300 Killer V (wife's bike), 60 cm 1989 Eddy Merckx Corsa Extra SLX
Mentioned: 0 Post(s)
Tagged: 0 Thread(s)
Quoted: 83 Post(s)
Liked 57 Times
in
36 Posts
...and let's not forget "evapotranspiration" whereby a substance goes directly to the gaseous phase without first going thru the liquid phase, like solid CO2, better known as "dry ice'.
#94
ignominious poltroon
Join Date: Jan 2022
Posts: 4,046
Mentioned: 3 Post(s)
Tagged: 0 Thread(s)
Quoted: 2241 Post(s)
Liked 3,443 Times
in
1,802 Posts
#95
Senior Member
Thread Starter
Join Date: Mar 2017
Location: San Clemente
Posts: 664
Bikes: 87 Bianchi X4, 95 Bianchi Ti Mega Tube, 06 Alan Carbon Cross X33, Gold plated Columbus AIR Guerciotti, 74 Galmozzi Super Competizione, 52 Bianchi Paris Roubaix.
Mentioned: 6 Post(s)
Tagged: 0 Thread(s)
Quoted: 259 Post(s)
Liked 540 Times
in
166 Posts
Back to the pot. I mistakenly waxed a little early as I had just gotten the 63 Specialissima and transferred the Garmin over mixing up my milage tally. Oh well. No big deal as it's so easy.
2100 total miles

Chain after 259 miles since last wax, nothing in between.

Cassette after 2100 miles with the wax. I never cleaned the cassette prior to starting the wax.

Chain after pouring a kettle of boiled water over it to clean.

Waxed and ready to roll.

2100 total miles

Chain after 259 miles since last wax, nothing in between.

Cassette after 2100 miles with the wax. I never cleaned the cassette prior to starting the wax.

Chain after pouring a kettle of boiled water over it to clean.

Waxed and ready to roll.

#96
Newbie
Join Date: Dec 2023
Posts: 13
Mentioned: 0 Post(s)
Tagged: 0 Thread(s)
Quoted: 7 Post(s)
Likes: 0
Liked 2 Times
in
1 Post
secret snake oil wax
just pain food grade paraffin wax with a 0.5 percent oil content,most candle supply places have this wax pretty cheap,you can get 1kilo blocks there,no need to add any secret sauce snake oil,they would be only very marginal gains,paraffin waxed chain is the smoothest chain ya gunna ride ever,its very popular down under now and some bicycle shops are doing waxed chains,that gulf wax actually beat all known lubes on the market when jason from friction facts tested gulf wax
#97
Newbie
Join Date: Dec 2023
Posts: 13
Mentioned: 0 Post(s)
Tagged: 0 Thread(s)
Quoted: 7 Post(s)
Likes: 0
Liked 2 Times
in
1 Post
chain lubes
Jason Smith from friction facts was the only guy in the world extensively testing bike lubes and wax,now there is Adam Kiren from Sydney who took over testing of modern lubes and chains,he runs each chain and lube tested until they cant run no more,its very extensive testing and water and sand is sprayed on each lubed chain in a controled setting so he can acuratly measure chain wear at each stage of the test,as far as i know its the most extensive testing ever done,Adam site is Zero friction cycling
#98
Newbie
Join Date: Dec 2023
Posts: 13
Mentioned: 0 Post(s)
Tagged: 0 Thread(s)
Quoted: 7 Post(s)
Likes: 0
Liked 2 Times
in
1 Post
waxing chains
If you add stuff like oils to paraffin it defeats the purpose of waxing a chain,you wax a chain to lubricate the chain but paraffin wax on its own also keeps out all the dust and grime,thats what mainly wears a chain out fast,that dirt,people often ask can you use candles,yes you can they do work but candles have a much higher oil content in them about around 7 percent, and the chain does get dirty faster,some home brews add beeswax,once again beeswax attracts dirt,once melted that paraffin wax gets inside those rollers no problems,and that is its main job,staying inside those rollers and keeping dirt and grime out which it does very well,is it a perfect lube?well its the best one I have ever used,the only downside is it may not protect the outside of the chain from rust if constanty riding in the wet weather but you can go buy a higher end nickle plated chain to fix that issue
#99
Mad bike riding scientist
Join Date: Nov 2004
Location: Denver, CO
Posts: 27,366
Bikes: Some silver ones, a red one, a black and orange one, and a few titanium ones
Mentioned: 152 Post(s)
Tagged: 1 Thread(s)
Quoted: 6219 Post(s)
Liked 4,220 Times
in
2,367 Posts
If you add stuff like oils to paraffin it defeats the purpose of waxing a chain,you wax a chain to lubricate the chain but paraffin wax on its own also keeps out all the dust and grime,thats what mainly wears a chain out fast,that dirt,people often ask can you use candles,yes you can they do work but candles have a much higher oil content in them about around 7 percent, and the chain does get dirty faster,some home brews add beeswax,once again beeswax attracts dirt,once melted that paraffin wax gets inside those rollers no problems,and that is its main job,staying inside those rollers and keeping dirt and grime out which it does very well,is it a perfect lube?well its the best one I have ever used,the only downside is it may not protect the outside of the chain from rust if constanty riding in the wet weather but you can go buy a higher end nickle plated chain to fix that issue
Candle wax does not have 7% oil in it. Bulk candle wax has from 1 to 1.5% oil in it from the supplier. “Oils” can be added for fragrance but 7% oil isn’t added because that much fragrance would drive a whole neighborhood out of their homes. Food grade wax has about 0.5% oil left in it. But oil addition or soft wax addition to paraffin to wax used for chain to make the wax more pliable might be as high as 7% but it would still be a solid. And a solid wax…even a soft one…is going to keep grit out of the chain that oil won’t.
__________________
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
#100
Newbie
Join Date: Dec 2023
Posts: 13
Mentioned: 0 Post(s)
Tagged: 0 Thread(s)
Quoted: 7 Post(s)
Likes: 0
Liked 2 Times
in
1 Post
wax
i do not pull up untried info from anywhere on the net,most of what i talk about is from trying stuff my self,you know nothing about waxing at all,if you think oil does not effect the wax you most likely dont know what your talking about and most likely have not used wax for many years,i do not parrot anything thank you very much,give me one good reason to use any oil with wax,oil cause dirt and grime to stick to the chain,another thing you are not correct about is the amount of oil in candle wax,it can be as high ad ten percent,go do your home before making stupid comments
Last edited by mikeonbicycle; 12-21-23 at 01:33 AM. Reason: update







