Does CO2 leak from tubes more rapidly than air?
#1
Member
Thread Starter
Does CO2 leak from tubes more rapidly than air?
Last Saturday I went for a ride and got a flat near the end of my ride, about 2 miles from home. Once I get home after having a flat, I normally "empty" the tube that flatted and then pump up with air. (Removing most of the CO2.)
Since I was headed out of town for work on Monday, I failed to do this - I figured I would do it when I got back. Well, I got back Thursday afternoon to a very deflated tube/tire.
With the rash of flats I have had lately I just assume I did not properly install the tube from my last flat or more bad luck.
A thought then occurred to me, could it just be that CO2 "leaks" from tubes more quickly than air?
Since I was headed out of town for work on Monday, I failed to do this - I figured I would do it when I got back. Well, I got back Thursday afternoon to a very deflated tube/tire.
With the rash of flats I have had lately I just assume I did not properly install the tube from my last flat or more bad luck.
A thought then occurred to me, could it just be that CO2 "leaks" from tubes more quickly than air?
#2
Advocatus Diaboli
Join Date: Feb 2015
Location: Wherever I am
Posts: 8,855
Bikes: Merlin Cyrene, Nashbar steel CX
Liked 1,613 Times
in
1,061 Posts
Yes
#4
Senior Member
Join Date: Feb 2006
Location: Sin City, Nevada
Posts: 2,914
Bikes: Catrike 700, Greenspeed GTO trike, , Linear LWB recumbent, Haluzak Horizon SWB recumbent, Balance 450 MTB, Cannondale SM800 Beast of the East
Likes: 0
Liked 244 Times
in
192 Posts
Don't obsess with it. Technically speaking, a molecule of CO2 which has two atoms of oxygen and one atom of carbon is larger than the two most prevalent molecules in ordinary air which is approximately 78% nitrogen and 21% oxygen. All of these molecules are infinitesimally small. What really determines the rate at which your tube loses pressure is how many tiny holes are in the rubber itself. There's no reason to empty the CO2 out of your tube and replace it with air. Also don't waste your money on having tires filled with nitrogen at a service station. That's the biggest scam since the invention of bottled water.
#5
Senior Member
Don't obsess with it. Technically speaking, a molecule of CO2 which has two atoms of oxygen and one atom of carbon is larger than the two most prevalent molecules in ordinary air which is approximately 78% nitrogen and 21% oxygen. All of these molecules are infinitesimally small. What really determines the rate at which your tube loses pressure is how many tiny holes are in the rubber itself.
#7
Member
Thread Starter
If it were really a question of molecules like tiny billiard balls bouncing off a rubber surface with some little holes then CO2 would actually stay inside the tube longer than either O2 or N2. But the chemistry comes into play. The CO2 has two carbon double bonds and these easily interact with the hydrocarbon compounds in rubber. As a result the CO2 molecules tend to stick to the rubber molecules and can gradually diffuse through the tube resulting in a gradual loss of pressure. The effect is easily noticed when you've used a CO2 cartridge to fill a tube instead of air.
Thanks for all the replies.
Jonathan
#8
Senior Member
If it were really a question of molecules like tiny billiard balls bouncing off a rubber surface with some little holes then CO2 would actually stay inside the tube longer than either O2 or N2. But the chemistry comes into play. The CO2 has two carbon double bonds and these easily interact with the hydrocarbon compounds in rubber. As a result the CO2 molecules tend to stick to the rubber molecules and can gradually diffuse through the tube resulting in a gradual loss of pressure. The effect is easily noticed when you've used a CO2 cartridge to fill a tube instead of air.
The advantages of carbon dioxide are that it's easily compressed into a liquid and very safe. Nitrogen would work better but it can't be easily compressed into a liquid except at very low temperatures.
Last edited by Hokiedad4; 09-16-17 at 05:12 AM.
#9
LBKA (formerly punkncat)
Join Date: Jan 2010
Location: Jawja
Posts: 4,299
Bikes: Spec Roubaix SL4, GT Traffic 1.0
Liked 960 Times
in
686 Posts
No, actually being a heavier molecule it will leak slower, but the thing to point out is that CO2 is thermally induced to pressure (under pressure) where air isn't in a noticeable way. So, if it's really hot your tube will hold more pressure for volume than if it's cold.
#10
Senior Member
[QUOTE=Hokiedad4;19865162]Carbon dioxide does not really interact with rubber. Butyl rubber is highly resistant to chemical interaction with most gases. But carbon dioxide diffuses through rubber much faster than air (relative permeability 2.9 versus 0.22 for air).
........Source of this "data"?
Thanks!
So you're not going to ride around with CO2 in the tires, but it'll get you home.
........Source of this "data"?
Thanks!
So you're not going to ride around with CO2 in the tires, but it'll get you home.
#11
Senior Member
It doesn't form new molecules by permanently bonding to the rubber, but the double bonds in CO2 can temporarily interact with the rubber hydrocarbon bonds. That's why the CO2 diffuses so readily through rubber - the temporary partial bonds makes for attraction between the CO2 and rubber molecules so they don't just bounce off each other.
#12
Senior Member
No, actually being a heavier molecule it will leak slower, but the thing to point out is that CO2 is thermally induced to pressure (under pressure) where air isn't in a noticeable way. So, if it's really hot your tube will hold more pressure for volume than if it's cold.
#13
Let's take this beyond theory and apply it to real world.
Assuming one leaks faster then the other. Are we talking 0.5 psi increase in loss of CO2 vs regular air over a month? Considering I usually put a few psi of air in my tires every week anyway..
Assuming one leaks faster then the other. Are we talking 0.5 psi increase in loss of CO2 vs regular air over a month? Considering I usually put a few psi of air in my tires every week anyway..
#15
Non omnino gravis
Definitely drops pressure faster, measured empirically, not though fancy maths. A tube pressured to ~100psi via a CO2 inflator will usually drop 10-20psi by the time I make it home, and be as low as 60-70psi by the time I get around to emptying it out the next morning.
But blissfully tubeless on all of our bikes now, so it's not really an issue anymore.
But blissfully tubeless on all of our bikes now, so it's not really an issue anymore.
#16
Occam's Rotor
Don't obsess with it. Technically speaking, a molecule of CO2 which has two atoms of oxygen and one atom of carbon is larger than the two most prevalent molecules in ordinary air which is approximately 78% nitrogen and 21% oxygen. All of these molecules are infinitesimally small. What really determines the rate at which your tube loses pressure is how many tiny holes are in the rubber itself. There's no reason to empty the CO2 out of your tube and replace it with air. Also don't waste your money on having tires filled with nitrogen at a service station. That's the biggest scam since the invention of bottled water.
#17
Senior Member
Technical Q&A with Lennard Zinn - Large molecules and short frames | VeloNews.com
"Alan Hills:
I’ve been told it was true by no less than (our local triathlon legend) Dave Scott. Wading through the web yields some insights on tire pressure loss from tires/tubes inflated with carbon dioxide (CO2) cartridges. Two polymers are used for bike tubes; latex rubber and butyl rubber (isobutylene rubber).
Butyl rubber dominates the market and is used for almost all tubeless tires and bike tubes as its permeability to air is incredibly low — butyl tubes have only 10 percent the leakage rates of natural latex rubber tubes.
Permeation by diffusion predicts gas leakage rates proportional to the inverse of the square root of their molecular weights. Using air as a reference the predicted leakage rates for common gases are: helium 2.7, air 1.0, nitrogen 1.02, oxygen 0.95, argon 0.85, carbon dioxide 0.81.
It turns out however that the leakage rate of CO2 is huge, and the reason is that it is actually soluble in butyl rubber and is thus not constrained to normal permeation loss, it can transfer straight through the bulk rubber resulting in severe tire pressure loss on the order of a single day. CO2 is not likely to be replaced by argon or other gases in refill cartridges, however, because CO2 is much more easily liquefied than other gases and can be contained in a moderate-pressure cartridge in a patch kit. An analogous cartridge holding N2 or argon (non-liquified gas) would be dangerous and would require a thick (and very heavy) steel-walled storage vessel. A reference dealing with CO2 transfer through latex rubber sheds light on the loss process."
"Alan Hills:
I’ve been told it was true by no less than (our local triathlon legend) Dave Scott. Wading through the web yields some insights on tire pressure loss from tires/tubes inflated with carbon dioxide (CO2) cartridges. Two polymers are used for bike tubes; latex rubber and butyl rubber (isobutylene rubber).
Butyl rubber dominates the market and is used for almost all tubeless tires and bike tubes as its permeability to air is incredibly low — butyl tubes have only 10 percent the leakage rates of natural latex rubber tubes.
Permeation by diffusion predicts gas leakage rates proportional to the inverse of the square root of their molecular weights. Using air as a reference the predicted leakage rates for common gases are: helium 2.7, air 1.0, nitrogen 1.02, oxygen 0.95, argon 0.85, carbon dioxide 0.81.
It turns out however that the leakage rate of CO2 is huge, and the reason is that it is actually soluble in butyl rubber and is thus not constrained to normal permeation loss, it can transfer straight through the bulk rubber resulting in severe tire pressure loss on the order of a single day. CO2 is not likely to be replaced by argon or other gases in refill cartridges, however, because CO2 is much more easily liquefied than other gases and can be contained in a moderate-pressure cartridge in a patch kit. An analogous cartridge holding N2 or argon (non-liquified gas) would be dangerous and would require a thick (and very heavy) steel-walled storage vessel. A reference dealing with CO2 transfer through latex rubber sheds light on the loss process."
#18
Advocatus Diaboli
Join Date: Feb 2015
Location: Wherever I am
Posts: 8,855
Bikes: Merlin Cyrene, Nashbar steel CX
Liked 1,613 Times
in
1,061 Posts
#19
LBKA (formerly punkncat)
Join Date: Jan 2010
Location: Jawja
Posts: 4,299
Bikes: Spec Roubaix SL4, GT Traffic 1.0
Liked 960 Times
in
686 Posts
Hi
Do you know why co/co2 detectors are placed within a foot of the ground?
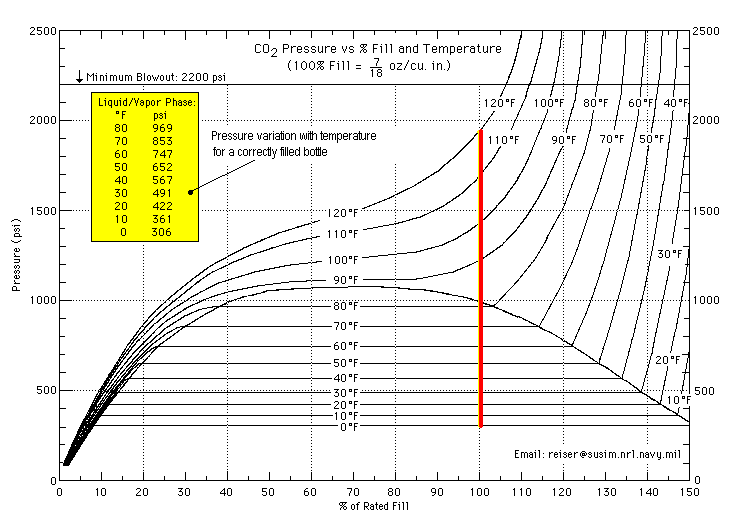
Are you familiar with this chart?
https://thehotpepper.com/uploads/mont...1463862733.gif
Last edited by Juan Foote; 09-18-17 at 07:43 AM.
#20
Senior Member
It still leaks out of butyl tubes faster than air. Keep searching for charts.
#21
LBKA (formerly punkncat)
Join Date: Jan 2010
Location: Jawja
Posts: 4,299
Bikes: Spec Roubaix SL4, GT Traffic 1.0
Liked 960 Times
in
686 Posts
That's a useful chart if you're interested in the temperature and pressure at which CO2 liquifies, however, I prefer a gas in my tires rather than a liquid. And my tires aren't rated for 400psi.
It still leaks out of butyl tubes faster than air. Keep searching for charts.
It still leaks out of butyl tubes faster than air. Keep searching for charts.
Edit- To add to that. A co2 powerlet has no gauge. We have no idea what our tire is actually at, it's just not "flat". Considering the volume of tire and size of the powerlet (which can be adjusted) it is safe to assume that most powerlets aren't actually putting the recommended amount of 80-110 (w/e) PSI in the tire in the first place. It's just good to ride home.
You get home, the tire cools, it looses a bit more of the unsure whether it was the right pressure that it had, and the perception is that your tire went flat faster.
Last edited by Juan Foote; 09-18-17 at 08:23 AM.
#22
Senior Member
The chart is meant for for co2 tanks/paintball applications. It carries over to any powerlet of co2 What it demonstrates though, is the volatility of it's pressure, even in aerosol state, at temperature. As we can see from this chart that difference is notably less at lower pressures but still there. I know I can feel five pounds difference in my little bitty tires.
Edit- To add to that. A co2 powerlet has no gauge. We have no idea what our tire is actually at, it's just not "flat". Considering the volume of tire and size of the powerlet (which can be adjusted) it is safe to assume that most powerlets aren't actually putting the recommended amount of 80-110 (w/e) PSI in the tire in the first place. It's just good to ride home.
You get home, the tire cools, it looses a bit more of the unsure whether it was the right pressure that it had, and the perception is that your tire went flat faster.
Edit- To add to that. A co2 powerlet has no gauge. We have no idea what our tire is actually at, it's just not "flat". Considering the volume of tire and size of the powerlet (which can be adjusted) it is safe to assume that most powerlets aren't actually putting the recommended amount of 80-110 (w/e) PSI in the tire in the first place. It's just good to ride home.
You get home, the tire cools, it looses a bit more of the unsure whether it was the right pressure that it had, and the perception is that your tire went flat faster.
#24
Non omnino gravis
Have you ever actually used a CO2 cartridge? There's this thing it does when it leaves the cartridge rapidly, which is it gets really, really, really cold. Unless the outside temperature is at or below freezing, a fixed flat filled with CO2 should be warmer and therefore at a higher PSI when getting home... if it weren't for the incontrovertible fact that CO2 diffuses right through the butyl rubber.
#25
LBKA (formerly punkncat)
Join Date: Jan 2010
Location: Jawja
Posts: 4,299
Bikes: Spec Roubaix SL4, GT Traffic 1.0
Liked 960 Times
in
686 Posts
Have you ever actually used a CO2 cartridge? There's this thing it does when it leaves the cartridge rapidly, which is it gets really, really, really cold. Unless the outside temperature is at or below freezing, a fixed flat filled with CO2 should be warmer and therefore at a higher PSI when getting home... if it weren't for the incontrovertible fact that CO2 diffuses right through the butyl rubber.
The other aspect is that you shouldn't be leaving CO2 (which is acidic) in a tube to be eating the rubber anyway.
Once again. Emergency get you home device.